Stable compounds of Gallium, Indium and Thallium in the +2 oxidation state
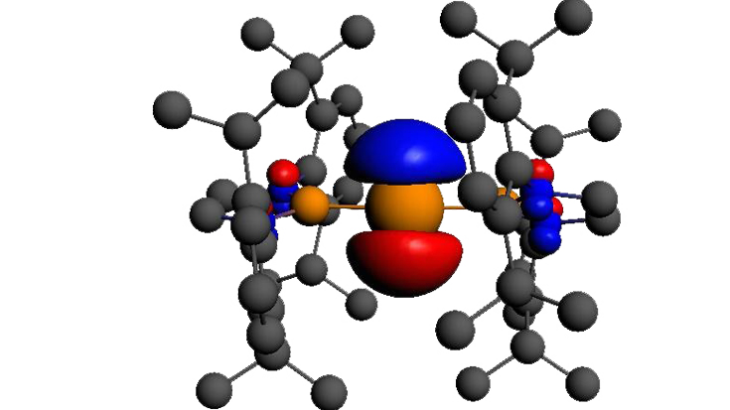
In a paper published in Nature Chemistry, a team of experimental chemists from the Universities of Oxford, Monash and Queensland, in collaboration with computational chemists at University College London, announced that they have succeeded in making thermally stable and robust compounds of gallium, indium and thallium in the +2 oxidation state. This is remarkable because the chemistry of these metals is dominated by the +1 and +3 oxidations states – and those in the +2 state are typically short-lived and highly reactive.
Professor Nik Kaltsoyannis, whose team in UCL’s Department of Chemistry ran the computational analysis that confirmed the discovery, explained this in terms that make more sense to the non-chemists among us. “Chemical bonds are formed because of atoms sharing electrons with each other – and the elements gallium, indium and thallium have three such electrons that they can, in principle, use to make bonds with other elements. In their chemistry, they typically use all three of them, or just one.
“So the compounds that we’re reporting on in this paper have a very unusual feature, in that gallium, indium and thallium have used only two of the three in a bond. And one unpaired atom remains on the metal atom in these compounds – which is highly unusual,” he says. Calculations by Kaltsoyannis’ team, using the IRIDIS High Performance Computing facility, were central to understanding the molecular structure and properties of the novel compounds – and to proving that they were in fact what they appeared to be. “What happened in this project – which is typical of many projects – is that someone made an unusual chemical, or in this case several unusual chemicals, that people have been trying to create for some time. In order to prove what it is that they’ve made, they run a lot of other experiments, and, independently calculations. If the results agree, then there’s a good chance that you’ve made what you think you’ve made,” he says.
“In the type of computational chemistry used here, we apply the fundamental principles of quantum mechanics, making very, very few assumptions and attempting to calculate from first principles … so if you get an answer that is comparable with an experimental result, you can be pretty confident in it. And one of the nice things about this particular paper is that there were quite a few experiments done, each measuring different things, and we were able to calculate pretty much all of them, and come up with answers that agree.”
While the UCL team ran many of the calculations using their own in-house computing system, the use of IRIDIS expands the potential research than can be done – and this particular research could not have been done without it, Kaltsoyannis says. “The IRIDIS facility just brings us the computing power we need. In this sort of modelling, some of the calculations take a very long time, and we simply didn’t have the computing resources without it. Compared to using the computers we have in the department, IRIDIS speeds things up by a factor of about 100.”
The gallium, indium and thallium project is complete for now. “It’s driven by the scientists in Oxford making these new and unusual molecules, so the extent to which the project goes forward is driven by them,” says Kaltsoyannis. “But we continue to use the IRIDIS facility, on a range of other projects that we have going in the research group. It’s an essential resource for us now,” he says.
The authors would like to acknowledge the work presented here made use of the IRIDIS High Performance Computing facility provided via the Science & Engineering South Consortium in partnership with STFC Rutherford Appleton Laboratory.

